the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Sources and behavior of perchlorate ions (ClO4−) in chalk aquifer of Champagne-Ardenne, France: preliminary results
Feifei Cao
Jessy Jaunat
Patrick Ollivier
Benjamin Cancès
Xavier Morvan
Daniel Hubé
Alain Devos
Nicolas Devau
Vincent Barbin
Pierre Pannet
Perchlorate () is an environmental contaminant of growing concern due to its potential human health effects and widespread occurrence in surface water and groundwater. Analyses carried out in France have highlighted the presence of in drinking water of Champagne-Ardenne (NW of France), with two potential sources suspected: a military source related to the First World War and an agricultural source related to the past use of Chilean nitrates. To determine the sources of in groundwater, major and trace elements, 2H and 18O, and ions and a list of 39 explosives were analyzed from 35 surface water and groundwater sampling points in the east of the city of Reims. ions were found in almost all sampling points (32 out of 35) with a max value of 33 µg L−1. concentrations were highest in groundwater ranging from 0.7 to 33 µg L−1 (average value of about 6.2 µg L−1) against from < 0.5 to 10.2 µg L−1 in surface water (average value of about 2.7 µg L−1). Most of the water samples showing high levels (> 4 µg L−1) were collected near a military camp, where huge quantities of ammunitions have been used, stored and destroyed during and after the First World War.
- Article
(527 KB) - Full-text XML
- BibTeX
- EndNote
Perchlorate () is an inorganic anion and a powerful oxidizer with high solubility and mobility in water. Relatively stable under ambient conditions, perchlorate may persist for many years, possibly decades, under typical surface water and groundwater conditions (Sturchio et al., 2014). Perchlorate salts are widely used as oxidizer in solid rockets fuel and as component of fireworks, pyrotechnics, flares and explosives (Urbansky, 1998). In addition, natural sources such as sodium nitrate deposits in Chile (Urbansky et al., 2001) and formation in the atmosphere have also been reported (Dasgupta et al., 2005). Perchlorate is an environmental contaminant of concern in water because of its potential ability to inhibit iodide uptake and to impact neurodevelopment, especially for foetuses and infants (Leung et al., 2010). Since 1997, perchlorate has been listed as a contaminant in drinking water monitoring programs in the USA (USEPA, 1998). The current health advisory level for is set at 15 µg L−1 based on the reference dose recommended by the US EPA. However, in many countries, drinking water standard for is yet to be defined. Many studies have underlined perchlorate contamination in water from numerous countries such as the USA, Canada (Backus et al., 2005), China (Wu et al., 2010), Japan (Kosaka et al., 2007) and India (Kannan et al., 2009).
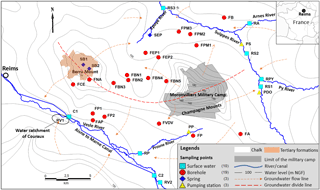
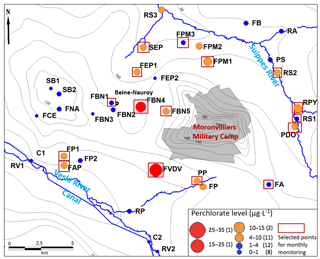
Figure 1Location of the study area, main rivers and sampling points (water level data from Rouxel-David et al., 2002).
In France, the interest of research on has increased since 2011 because of the discovery of contamination in several drinking water resources in South-Western France, in the Paris Basin and in North-Eastern France (Lopez et al., 2015). In the Champagne-Ardenne region (NE of France), the measurement campaigns carried out by the Regional Health Agency (ARS) in 2014 have showed high levels of ions (> 15 µg L−1) in raw water resources intended for human consumption. Two potential sources of in groundwater are suspected: military and agricultural. Indeed, the Champagne-Ardenne region was highly marked by the events of the First World War that may have emitted large quantities of perchlorates and other explosives in the environment, and France has imported, between 1875 and 1920, large quantities of natural nitrates as fertilizers from Chile, which are particularly rich in ions. The study site in an intensive agricultural area, thereby it is concerned with the use of these Chilean nitrates.
The objectives of this study are (1) to identify the sources of and other explosives in groundwater and to establish links between their occurrence and the type of human activities (military and/or agricultural), (2) to understand the transfer mechanisms of in the Champagne chalk aquifer, with studies on flow and mineralization processes in the aquifer. A study area has been selected in the Champagne-Ardenne region for a continuous monitoring of water chemistry to observe a temporal and spatial evolution of the concentrations of the molecules concerned. In this paper, we will focus on the results of the 2 screening measurement campaigns carried out in 2017, with the purpose to obtain a water chemical map of the study area and to proceed a further monthly monitoring over the next 2 years.
2.1 Study area
2.1.1 Location
The study area is located in the east of Reims (NE of France; Fig. 1) and covers approximately 600 km2 between the Suippe River (as the northern and western boundary) and the Vesle River (as the southern boundary), where some water catchments intended for drinking water supply are significantly impacted by the presence of ions.
It corresponds to an agricultural zone (traditional crops of wheat and beet, for which Chilean nitrates were largely used to increase the production until the 1920s) located on the former battlefield of the First World War (military camp of Moronvilliers and the surrounding trench areas). In addition to some forests covered at the Berru Mount and the Champagne Mounts, the whole zone is used as agricultural lands.
2.1.2 Hydrogeological context
The aquifer of the study area is the unconfined Champagne chalk aquifer, which is a crucial water resource of the region. In some areas, the chalk is partially covered with Tertiary (the Berru Mount; Fig. 1) or Quaternary formations (the valleys of the Vesle River, the Suippes River and their tributaries).
The total porosity of the Chalk is about 40 %, with only 1 to 5 % related to the effective porosity (Rouxel-David et al., 2002; Vachier et al., 1987). The Champagne Mounts constitute the major reliefs where the transmissivity ranges from 10−6 to 10−5 m2 s−1. In the valleys, these values are much higher, ranging from 10−2 to 0.3 m2 s−1. The Darcy velocity of the water flow in the saturated zone varies between 0.16 m yr−1 at the reliefs and 315 m yr−1 in valleys (Rouxel-David et al., 2002).
Table 1Information on sampling locations and perchlorate concentrations. The values in bold are the maximum concentrations of perchlorate in June and September. The symbol “X” represents the points selected for monthly monitoring.
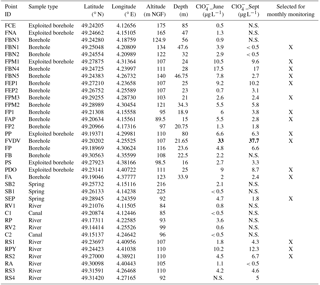
N.S.: Non sampled points.
The study area is divided into two parts by the groundwater divide line across the summit of Berru Mount and the Champagne Mounts, which delimits the Vesle River watershed in the south and the Suippe River watershed in the north. According to water levels (Rouxel-David et al., 2002), the chalk aquifer of the study area is generally drained by rivers except the water catchment of Couraux (Fig. 1) which supplies the drinking water for the Reims area. Due to the drawdown of groundwater level caused by pumping, the catchment is partially fed by river water, especially during low water period. The precipitation constitutes the only recharge of the Champagne chalk aquifer, mainly between September and April.
2.2 Sampling
Two chemical screening campaigns have been carried out in June and September 2017. In June, 25 groundwater (22 boreholes and 3 springs) were collected. All the selected sampling points are situated on areas both agricultural and concerned by military activities. Ten points of surface waters, distributed in the Aisne to Marne Canal, Vesle River, Suippes River and their tributaries (Table 1 and Fig. 1) were also sampled to take into account the groundwater/surface water exchanges in the diffusion of the molecules. In September, a total of 25 water samples were collected (Table 1). The points with non-detectable or very low levels were not sampled and a new one has been added in the Suippes River. The physicochemical parameters (pH, conductivity, temperature, alkalinity, dissolved oxygen and redox potential) were measured at the sampling points.
2.3 Instrumental analysis
The quantification of was performed using a two-dimension ion chromatography on an ion chromatography (DIONEX ICS 2000) at BRGM (Orléans, France), with a detection limit of 0.5 µg L−1. Quantitative analysis of cations (Ca2+, Mg2+, Na+ and K+) and anions (, , Cl−, F−) was done by ICP Optical Emission Spectrometry (ICAP 6300, THERMO) and Ion Chromatography system (DIONEX ICS 2000) respectively at GEGENAA (Reims, France). The analysis of 39 explosives (e.g. TNT, trinitrophenol, trinitronaphthalene) was performed by the company Envilytix GmbH (Wiesbaden, Germany).
3.1 Major ions
Calcium and bicarbonate are the predominant ions in water, which is consistent for groundwater in Chalk aquifer. However, spatially heterogeneities are present not only on Ca2+ and but also on Na+, Cl−, and contents. This may be due to human activities and/or mineralization conditions of the aquifer, assumptions to be confirmed.
3.2 Perchlorate and explosives
The two screening campaigns show almost similar results for concentrations (Table 1). Perchlorate ions were found in almost all the samples, and the mean concentrations in groundwater and surface water samples were 6.2 and 2.7 µg L−1, respectively. 15 among the 35 water points (43 %) present levels higher than 4 µg L−1 (concentration beyond which infants should not consume water according to French health authorities advices) and 1 sampling point exhibits concentrations higher than 15 µg L−1 (US EPA reference dose). Twenty sampling points (57 %) show levels lower than 4 µg L−1.
Most of the sampling points showing concentrations above 4 µg L−1 are found around the military camp of Moronvilliers, where large quantities of ammunition were used, stored and destroyed during and after the Great War. The maximum level is 33 µg L−1 (FVDV, south of military camp of Moronvilliers; Fig. 2). According to historical data, this sampling point is located at a former military airfield used during the Great War. In addition, the military camp of Moronvilliers is located upstream of this point, representing a potential source of . Samples from FBN4, FVDV and FP show also chlorate () levels slightly higher than the other samples.
The sampling points showing levels lower than 4 µg L−1 are mainly located on the Berru Mount, in the Vesle River and the Aisne to Marne Canal. concentrations in the canal are less than 0.5 µg L−1. Since water in the canal is not (or little) related to the chalk aquifer, it verified the fact that in river waters come from the exchanges with groundwater. concentrations in rivers are partly diluted by upstream water and drainage of low-level parts of the aquifer so that generally lower levels of than those in groundwater are measured.
However, some surface waters show significantly higher levels in (> 10 µg L−1), such as the RPY (the Py River) and RS2 (downstream of the Py in the Suippe River; Fig. 2 and Table 1). sources exist thus in the Py catchment area. As the point RS1 (upstream of the Py, in the Suippes river) has a low level of (< 4 µg L−1), the high level on RS2 is most likely from the contribution of the Py river. Indeed, the Py River is originated from downstream of the military camp of Suippes, potentially representing a source of .
Note that explosives have not been detected in surface and groundwater samples, which may be due to their low persistence in soil and water.
Sixteen sampling points were selected based on the results of the 2 sampling campaigns for a monthly monitoring over the next 2 years (Fig. 2). Sampling points showing high concentrations were selected. Although the levels on FBN1 and FA were low, these two sampling points will also be collected to ensure a consistent distribution of sampling points in the west and the southeast of the study area.
The preliminary results of the 2 sampling campaigns allowed us to obtain the chemical facies of groundwater, the levels and distribution of perchlorate ions in the study area. Most of sampling points showing high levels of are located around the military camp of Moronvilliers, representing a potential source of perchlorate. That must be confirmed with further studies.
Over the next 2 years, measurements will be performed on 16 sampling points in the study area to determine the contents of major and trace elements, and ions in waters and stable isotopes of water molecule (2H and 18O). In parallel, the measurement of the isotopic signature of oxygen and chlorine in will make it possible to precise the sources of perchlorates (agriculture and/or military). A better understanding of the chalk aquifer properties and flow mechanisms will be reached by the measurement of the residence time of groundwater and the monthly monitoring of the chemical composition of waters. Finally, this multi-tracer approach will allow us to predict the spatial and temporal evolution of concentrations, and thus offer innovative information to make appropriate recommendations in terms of water management.
The data supporting the findings of this study came from the site study results and are available within the article.
The authors declare that they have no conflict of interest.
This article is part of the special issue “Innovative water resources management – understanding and balancing interactions between humankind and nature”. It is a result of the 8th International Water Resources Management Conference of ICWRS, Beijing, China, 13–15 June 2018.
This study is financially supported by the BRGM, the Agence de l'Eau
Seine-Normandie, the Region Grand-Est, the Grand-Reims Metropolis and the ARS
Grand-Est.
Edited by: Depeng Zuo
Reviewed by: two anonymous referees
Backus, S. M., Klawuun, P., Brown, S., D'sa, I., Sharp, S., Surette, C., and Williams, D. J.: Determination of perchlorate in selected surface waters in the Great Lakes Basin by HPLC/MS/MS, Chemosphere, 61, 834–43, 2005.
Dasgupta, P. K., Martinelango, P. K., Jackson, W. A., Anderson, T. A., Tian, K., Tock, R. W., Tock, R. W., and Rajagopalan, S.:: The origin of naturally occurring perchlorate: the role of atmospheric processes, Environ. Sci. Technol., 39, 1569–1575, 2005.
Kannan, K., Praamsma, M. L., Oldi, J. F., Kunisue, T., and Sinha, R. K.: Occurrence of perchlorate in drinking water, ground water, surface water and human saliva from India, Chemosphere, 76, 22–6, 2009.
Kosaka, K., Asami, M., Matsuoka, Y., Kamoshita, M., and Kunikane, S.: Occurrence of perchlorate in drinking water sources of metropolitan area in Japan, Water Res., 41, 3474–82, 2007.
Leung, A. M., Pearce, E. N., and Braverman, L. W.: Perchlorate, iodine and the thyroid, Best Pract. Res. Cl. En., 24, 133–141, 2010.
Lopez, B., Vernoux, J. F., Neveux, A., Barrez, F., and Brugeron, A.: Recherche des origines possibles de la pollution en perchlorates impactant des captages en eaux souterraines du territoire Nemours-Bourron, Final report, BRGM/RP-64840-FR, 2015.
Rouxel-Davis, E., Cordonnier, G., and Dachy, S.: Synthèse des études menées sur le bassin versant de Couraux (Marne), BRGM, 2002.
Sturchio, N. C., Beloso Jr., A., Heraty, L. J., Wheatcraft, S., and Schumer, R.: Isotopic tracing of perchlorate sources in groundwater from Pomona, California, Appl. Geochem., 43, 80–87, 2014.
Urbansky, E. T.: Perchlorate chemistry: implications for analysis and remediation, Bioremediat. J., 2, 81–95, 1998.
Urbansky, E. T., Brown, S. K., Magnuson, M. L., and Kelty, C. A.: Perchlorate levels in samples of sodium nitrate fertilizer derived from Chilean caliche, Environ. Pollut., 112, 299–302, 2001.
USEPA: Drinking water contaminant list, EPA document No. 815-F-98-002, GPO, Washington, DC, 1998.
Vachier, P., Dever, L., and Fontes, J. C.: Mouvements de l'eau dans la zone non satureìe et alimentation de la nappe de la craie de champagne (France): Isotope Techniques in Water Resources Development, IAEA Conf., Vienna, 30 March–3 April 1987, 367–379, 1987.
Wu, Q., Zhang, T., Sun, H., and Kannan, K.: Perchlorate in tap water, ground water, surface waters and bottled water from China and its association with other inorganic anions and with disinfection byproducts, Arch. Environ. Con. Tox., 58, 543–50, 2010.