the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Application of Geochemical and Isotopic Tools to Investigate Water Recharge and Salinization in a Coastal Phreatic Aquifer Suffering Severe Natural and Anthropogenic Constraints: Case of the Mornag Aquifer, NE Tunisia
Mohsen Ben Alaya
Jean-Denis Taupin
Mohamed Haythem Msaddek
Inès Ayari
Nicolas Patris
Najet Chaabene
Baya Toumi
Fetheddine Melki
The determination of the origin of salinity in the superficial aquifer of Mornag (NE Tunisia), and the understanding of its hydrological and geochemical behaviours related to severe natural and anthropogenic constraints, were approached by the combined study of chemical elements and stable isotopes (2H and 18O). This study indicates that: (1) the high salinities of the superficial aquifer of Mornag are mainly explained by the dissolution/precipitation processes of evaporite minerals in the aquifer formation, (2) the present-day recharge during rainwater infiltration brings downward a high content of nitrates and other dissolved salts, (3) infiltration of untreated sewage from the main urban areas contaminates the aquifer, (4) two other sources of dissolved salts in groundwater exist, favoured by the intensive exploitation of the phreatic aquifer. The first one is due to mineralised water infiltration from Meliane Wadi where activities, mainly a cement factory, discharge their wastewater. Intrusion of marine saltwater is the second source of salinity caused by aquifer over-exploitation. This hypothesis is supported by the high chloride concentration (>122 m e.q. L−1), ratios (>1.8 ‰) and the piezometric level lower than sea level. On the other hand, the artificial recharge with low mineralization waters by the Mejerda-Cap Bon Canal and the natural recharge in the valley of wadi El Hma, contribute to a dilution of groundwater. The freshwater/saltwater mixing causes geochemical interactions modifying the water chemistry: cationic exchange, precipitation phenomena. Isotopic tools (2H and 18O) show that the water of this aquifer system has been recently recharged by direct infiltration from the boundary and in the valley of wadis.
- Article
(4803 KB) - Full-text XML
- BibTeX
- EndNote
SGD 6; field observation
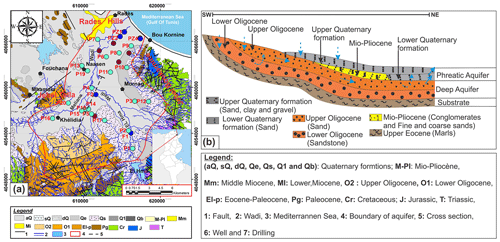
Groundwater resources of the Mediterranean coastal plains and more particularly the phreatic ones in the southern bank of the basin (Middle East and North Africa) show a qualitative and quantitative deterioration developing in time (Edmunds and Droubi, 1987; Custodio and Bruggeman, 1987; Richter and Kreitler, 1993; Vengosh and Rosenthal, 1994; Vengosh et al., 1999). This study concerns he Phreatic coastal Aquifer of Mornag is located in the Northeast of Tunisia (Fig. 1), where a semiarid Mediterranean climate prevails. The dry season is pronounced and this exacerbates the situation, given pumping rates higher usually coincides with the period of least recharge. Indeed, the coastal plain of Mornag hosts a large number (more than 1500) of wells with depths varying from a few meters to nearly 250 m. Some wells are used for drinking water, and little for industry, and around 97 % of the water is used for irrigation. depletion of the groundwater resources and degradation of their quality. The total water extraction in the These exploitation rates, which are required to meet the increasing demands of the current agricultural practices, exceed the natural replenishment of these basins and had led to basin is estimated by the local water authorities as 24.44 million cubic meters per year. On average, groundwater extraction in the basin exceeds recharge by an estimated 7 million cubic meters annually (DGRE, 2017). This over-pumping of the alluvial aquifer has resulted in water level declines ranging from 2.59 to 4.75 m yr−1 during the past three decades. The water quality is highly variable and in some areas reaches high salinity levels exceeding 5 g L−1. Evaluation of the mechanisms that cause the degradation of the groundwater quality and water recharge in costal phreatic aquifer in Mornag basin is the focus of this work.
This study concerns the coastal phreatic aquifer of Mornag basin of northeast Tunisia (Fig. 1a). This region has a semiarid climate, with extreme temperatures and rainfall variations with an average annual temperature and rainfall of 20 °C and 454 mm yr−1, respectively. The age of geological formations cropping out in Mornag basin extends from the Triasic to Quaternary. The catchment area is composed of gypsum, halite, limestone, dolomite, sand, sandstone, clayey sand and conglomerates. A representative West–East cross section of the study area is shown in Fig. 1b. The costal phreatic aquifer is located in the Quaternary layer system formed by sand, clayey sand and conglomerates. The phreatic aquifer is characterized by moderate transmissivity ranging from 1 to 10−3 m2 s−1.
Twenty-five groundwater samples from Mornag coastal phreatic aquifer were sampled in September–October 2016 from pumping wells (Fig. 1a). Several analyses, water temperature, pH, and electrical conductivity (EC) were carried out on-site. All samples were filtered directly in the field through 0.45 µm membrane filters, stored in high-density polyethylene bottles of 250 mL, and kept at 4 °C. Stable isotope composition of water samples were analysed at the Laboratory LAMA of HydroSciences Montpellier. Major chemistry and trace elements were determined by several methods (Titration, Ion Chromatography and flame photometry inductively at several Laboratorys of National Institute of Research and Physical-Chemical Analysis (INRAP-Tunisia). All samples showed an ionic unbalance smaller than ±5 % (Mandel and Shiftan, 1981).
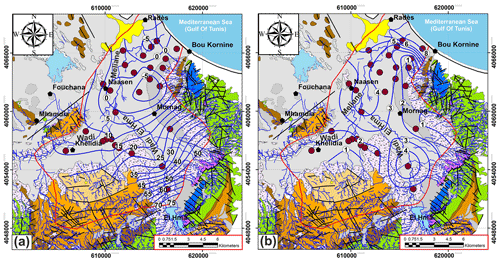
4.1 Piezometry
Piezometric surveys of phreatic aquifer performed in September–October 2016 (Fig. 2a):
-
The aquifer is recharged by direct infiltration and its higher limits is at Jebel Boukarnine in Southeast, Khlidia region, Jebel Tella and Rades Hill;
-
The discharge limits coincide with the Mediterranean shore line,
-
The groundwater flow is mainly toward the south-east and north-west to the middle of basin and may be locally disturbed by piezometric depressions (−5 m below sea level) due to the intensive exploitation,
The piezometric gradient varies from 7 ‰ to 12 ‰ in the upstream zone and 3.57 ‰ to 6 ‰ in the downstream zone.
4.2 Chemical Tracing
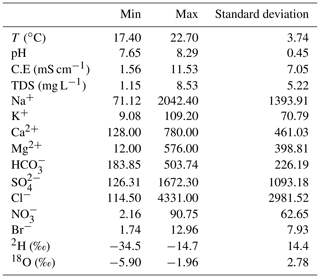
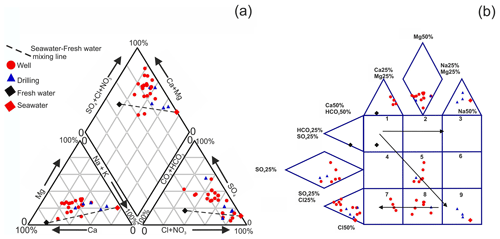
The measured physical and chemical parameters showed large spatial variations (Table 1). The electrical conductivity varied between 1748 and 11 530 µS cm−1 and the salinity between 1.15 and 8.53 g L−1 (Fig. 2b). The results of chemical analyses indicated most of the Mornag groundwater samples show similar water types: SO–Cl−–Ca2+ and Cl−–SO–Ca2+ (Fig. 3a).
The expanded Durov diagram was used (Fig. 3b) to identify processes and reaction paths such as mixing, ion exchange and dissolution affecting groundwater composition. Box 5, where a part of samples is present, is close to conservative mixing waters. However, the samples belong to Boxes 7 and 8, are characterized by mixing and reverse ion exchange affecting the composition of water sampled. A maximum increase in the salinity should produce water in box 9, where we see a mixture with seawater
Some samples present a depletion in Na+ content with respect to Cl− concentration, probably reflecting the cation exchange reactions leading to adsorption of Na+ on clay minerals belonging to aquifer formations and simultaneous releasing of Ca2+ ions (Fig. 4a). On the other hand, the remaining part of the samples shows a more obvious loss of Ca2+ with respect to SO. This may be the result of calcite precipitation controlled by gypsum dissolution which must be to maintain saturation or oversaturation. In fact, bicarbonates formed by CO2 dissolution is balanced by Na+ and Ca2+ released from clay minerals (Andrews et al., 1994). The cation exchange process was confirmed through the relation characterized by a slope of −1 (Fig. 4b) traced by the position of the samples (Garcia et al., 2001). Without exchange, all analytical points should lie close to the origin.
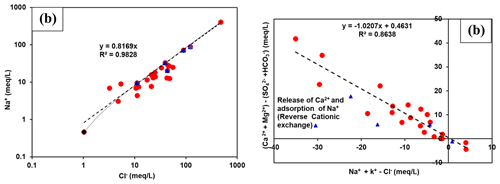
Figure 4(a) Relationships between (a) and Relationship between [(Ca Mg2+) − (HCO SO] and (Na K+ − Cl−) reflecting the cation exchange processes.
The majority of these waters sampled, are marked by relatively high chloride concentrations and the ratios varied between 0.45 ‰ and 8.97 ‰. Five wells show a ratio similar to that of seawater (1.3 ‰ to 2 ‰). Other waters with a high content (>1000 mg L−1) and ranging from 0.45 ‰ to 1 ‰ could be linked to leaching of the Triassic salty evaporitic formations, outcropping in southern boundaries of basin. Waters with a relatively low chloride content (<1000 mg L−1) and ranging from 2 ‰ and 8.97 ‰, according to the classification proposed by Alcalá and Custodio (2008) or Zabala et al. (2015), a contribution of atmospheric precipitation and agricultural activities on groundwater salinity. However, it can be suggested that the values observed in these wells can only be the consequence of intensive use of fertilizers in this part of the study area. An additional enrichment in is also found as a result of industrial pollution like this cement factory, discharge their wastewater in Meliane Wadi.
The excessive use of inorganic fertilizers and other substances (pesticides, fungicides, etc.) may introduce anthropogenic toxic elements into the groundwater. In Mornag coastal plain, nitrogen fertilizers are used for the cultivation of vegetable crops. Among all sampled water from the coastal phreatic aquifer of Mornag, 6 showed concentrations of nitrates ranging from 50.13 to 90.75 mg L−1. Nitrate pollution affects shallow wells in the same way as deeper ones. This may be explained by a high permeability of the aquifer infiltration zone.
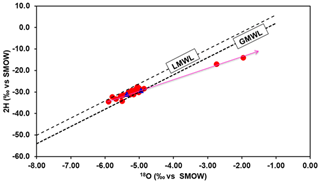
Stable isotopes composition of water samples from all wells collected in the phreatic coastal aquifer of Mornag suggests the presence of recently recharged water (Fig. 5a). Oxygen-18 and deuterium values in this area range, respectively, from −5.90 ‰ to −1.96 ‰ versus V-SMOW and from −34.4 ‰ to −14.7 ‰ versus V-SMOW. The mean isotopic values are, respectively, −5.04 ‰ and −29.3 ‰ versus V-SMOW for δ18O and δ2H, close to the values found in regional precipitations (δ18O ‰ and δ2H = −25.1 ‰) (Celle-Jeanton et al., 2001) in the humid season suggesting the groundwater was linked to current rainfall origin. The highest values come from the upstream part of the plain, near Hma dam; in this zone, the variability is high. Elsewhere, the isotopic composition of the aquifer is more homogeneous.
A combination of piezometric data, major elements geochemistry, trace elements and stable isotopes has provided a preliminary comprehensive understanding of the hydrodynamic mode of coastal phreatic aquifer Mornag and has allowed to highlight mechanisms linked to observed mineralization and reduced water quality. The following conclusions can be drawn: (1) salinization processes of groundwater are due to washing away of the evaporate levels in aquifer formation and contamination from the surface by chlorides and nitrates, and (2) isotopic tracing (18O and 2H) provide a mean for understanding the specification and location of the groundwater recharge. The phreatic aquifer is very vulnerable and only the application of protective measures will allow a better management of this resource for future generations. This study proposes a conceptual model of flow circulation allowing in a second close step to present a numerical model which will take account possible scenarios of groundwater evolution.
The code supporting the findings of this study is available from the corresponding author on request.
All geochemistry data are not dealt with and Table 1 gives the mean of various physico-chemical parameters measured, the chemical data will be the subject of another paper.
MBA carried out the investigations and interpretations of this study in collaboration with scientific supervision of JDT and NP performed the stable isotope analyses. MBA prepared the manuscript with contributions from all co-authors.
The contact author has declared that none of the authors has any competing interests.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the special issue “IAHS2022 – Hydrological sciences in the Anthropocene: Variability and change across space, time, extremes, and interfaces”. It is a result of the XIth Scientific Assembly of the International Association of Hydrological Sciences (IAHS 2022), Montpellier, France, 29 May–3 June 2022.
The authors gratefully acknowledge the contributions of the staff members of Ben Arous Water Resources Division/Agriculture Ministry, for their help during field work. We also thank the technical staff of the Laboratory LAMA of HydroSciences Montpellier and Laboratory of National Institute for Research and Physico-Chemical Analysis (INRAP) for their help and assistance during laboratory analyses.
This paper was edited by Christophe Cudennec and reviewed by two anonymous referees.
Alcalá, F. J. and Custodio, E.: Using the ratio as a tracer to identify the origin of salinity in aquifers in Spain and Portugal, J. Hydrol., 359, 189–207, https://doi.org/10.1016/j.jhydrol.2008.06.028, 2008.
Andrews, J. N., Fontes, J. C., Aranyossy, J. F., Dodo, A., Edmunds, W. M., Joseph, A., and Travi, Y.: The evolution of alkaline aquifer of the Irhazer Plain, Niger, Water Resour. Res., 30, 45–61, https://doi.org/10.1029/93WR02226, 1994.
Bujalka, P., Johan, K., Krivy, M., Rakus, M., and Vacek, J.: Carte géologique de Grombalia à l'échelle 1:50 000, Service Géologique de Tunisie, 1971.
Celle-Jeanton, H., Zouari, K., Travi, Y., and Daoud, A.: Caractérisation isotopique des pluies en Tunisie, Essai de typologie dans la région de Sfax, C. R. Acad. Sci. Paris, 333(IIA), 625–631, https://doi.org/10.1016/S1251-8050(01)01671-8, 2001.
Custodio, E. and Bruggeman, G. A.: Groundwater problems in coastal areas, UNESCO, Studies and Reports in Hydrology, UNESCO Press, París, 45, 576 pp., ISBN 978-92-3-102415-3, 92-3-102415-9, 1987.
Direction Générale des Ressources en Eau (DGRE): Annuaire d’exploitation des nappes en Tunisie, Direction Générale des Ressources en Eau (DGRE), 2017.
Edmunds, W. M. and Droubi, A.: Groundwater salinity and environmentalchange, in: International Symposium on Isotope Techniques in the Study of Environmental Change, Keynote Paper, STI/PUB/1024, IAEA, Vienna, Austria, 503–518, ISSN: 0074-1884, 1997, 1987.
Garcia, M. G., Del Hidalgo, M., and Blesa, M. A.: Geochemistry of groundwater in the alluvial plain of Tucumàn province Argentina, Hydrogeol. J., 9, 597–610, https://doi.org/10.1007/s10040-001-0166-4, 2001.
Jauzein, A.: Carte géologique de Bir M'Cherga à l'échelle 1:50 000, Service Géologique de Tunisie, 1957.
Mandel, S. and Shiftan, Z. L.: Groundwater resources investigation and development, Academic Press Inc., New York, 274 pp., ISBN 9780323157827, 1981.
Richter, B. C. and Kreitler, C. W.: Geochemical Techniques for Identifying Sources of Groundwater Salinization, CRC Press, Boca Raton, FL, USA, 272 pp., ISBN 9781566700009, 1993.
Vengosh, A. and Rosenthal, A.: Saline groundwater in Israel: its bearing on the water crisis in the country, J. Hydrol., 156, 389–430, https://doi.org/10.1016/0022-1694(94)90087-6, 1994.
Vengosh, A., Spivack, A. J., Artzi, Y., and Ayalon, A.: Boron, strontium and oxygen isotopic and geochemical constraints for the origin of the salinity in groundwater from the Mediterranean Coast of Israel, Water Resour. Res, 35, 1877–1894, https://doi.org/10.1029/1999WR900024, 1999.
Zabala, M. E., Manzano, M., and Vives, L.: The origin of groundwater composition in the pampeano aquifer underlying the Del Azul Creek basin, Argentina, Sci. Total Environ., 518–519, 168–188, https://doi.org/10.1016/j.scitotenv.2015.02.065, 2015.